The characteristics of organisms are "variety."
Variety!
Since the body of the eukaryotic is made of the same eucaryocyte as us the human being, the cell-biology investigation also deepens a human being's realization. At this laboratory, we are studying eukaryotics, such as the yeast which can eat D-amino acids, and algae which synthesizes an organic compound from CO2 using light energy.
An archea and an eubacterium are in a prokaryotic. The microbe lives the inside of the abyssal which a photic does not reach, frozen ground, or near a volcanic burner, etc. We are cultivating the bacteria which likes a sulfur or 100℃.
It is a difficult but pleasant investigation that how and where does a microbe alive. A microbe makes a thing necessary for a human being, or decomposes an unnecessary thing. For recycling of an element, the microbe is bearing the important part on the earth.
A microbe makes a thing necessary for a human being, or decomposes an unnecessary thing. For recycling of an element, the microbe is bearing the important part on the earth.
The archea live in the place over 100oC
Bacterium likes sulfur
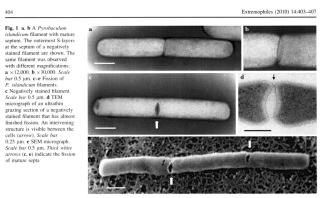
 Starkeya novella
Starkeya novellaStarkeya novella
Alanine Racemase from Green Alga
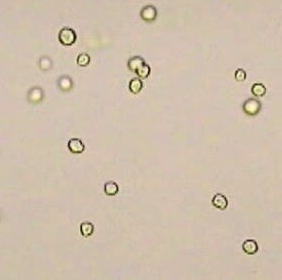 Photo: Chlamydomonas reinhardtii
Photo: Chlamydomonas reinhardtiiChlamydomonas is a genus within the unicellular green microalgae. This green microalga has recently been used as a model system in many fundamental studies in cell biology and molecular biology. In Phaeophyta, it was reported that the free D-alanine content was high in Hizikiafusiformis, Heterochordariaabietina, and Sargassumnigrifolium (Nagahisa et al., 1995). On the other hand D-aspartate was detected in both some fresh water microalgae and some marine diatoms, while D-alanine was only present in the latter (Yokoyama et al., 2003). There have been no studies, however, carried out regarding the presence of D-amino acids on Chlamydomonas reinhardtii. Studies of growth responses of Arabidopsisthaliana to D-alanine and D-serine show that these compounds inhibit growth even at quite low concentrations and are metabolized by recombinant D-amino acid oxidase into a non-toxic product (Erikson et al., 2004). Our preliminary experiment showed that C.reinhardtii could grow well in liquid medium containing 0.1% D-alanine, leading us to investigate enzymes that detoxify D-alanine.
In plants, only a few studies regarding the presence of an amino acid racemase have been conducted. Two studies on wheat and tomato tryptophan racemase were performed to examine characters such as activation by osmotic stress (Rekoslavskaya et al., 1997; Kopytina et al., 1998). Recently Yokoyama et al. reported alanine racemase activity in a diatom Thalassiosira sp. along with some of its properties (Yokoyama et al., 2005). In the present study, we report the partial purification and characterization of an alanine racemase from C.reinhardtii. We also demonstrate that D-alanine has no inhibitory effect on growth and induces alanine racemase activity in this green alga.
Chlamydomonas reinhardtii, a unicellular green microalga, could grow to a stationary phase having optical density of 2.0-2.5 at 750 nm in Tris-acetate-phosphate (TAP) medium containing 0.1% D-alanine. D-alanine has no inhibitory effect on growth and induced alanine racemase activity 130-fold more than without D-alanine in the green alga. Although C.reinhardtii cultured in the TAP medium showed alanine racemase activity, the content of free D-alanine was only 0.14%. The enzyme was partially purified by ammonium sulfate fractionation followed by three kinds of liquid chromatography using DEAE Toyopearl, Phenyl Sepharose, and TSK G3000 SWXL columns. The specific activity for L-alanine of the partially purified alanine racemase was 3.8 micro-mol/min/mg. The molecular weight of the enzyme was determined to be approximately 72,000 by gel filtration. The enzyme showed a maximum activity at 45oC and pH 8.4 and requires pyridoxal 5'-phosphate as a coenzyme.
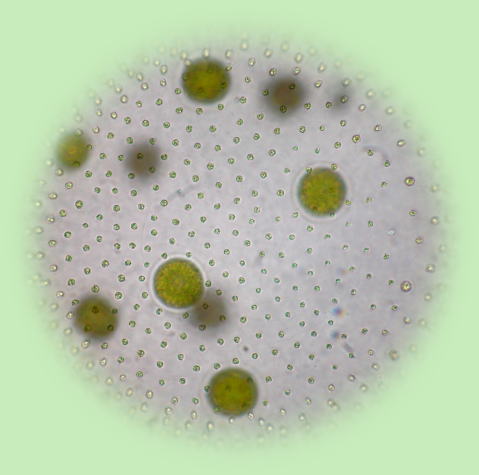
Photo: Volvox carteri
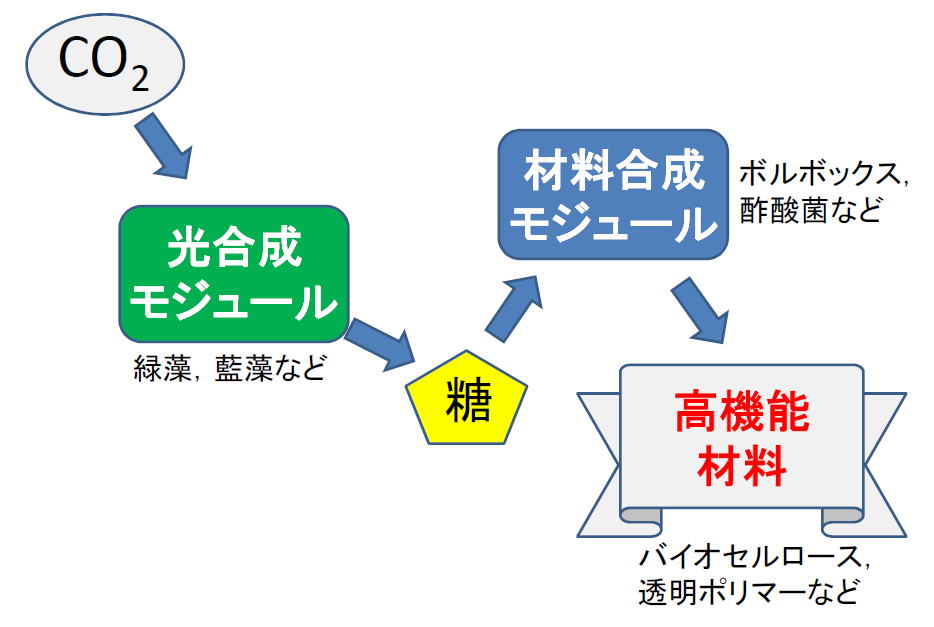
Making fine organic compounds by alga and cyanobacteria using CO2
Red yeasts make oxidase for acidic D-amino acids
Rhodotorula glutinis)
Application of Phage-display Technique
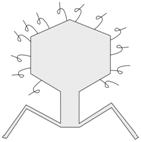
Bacteriophage with artificial hair